Autoimmune Disease Risk Is Elevated by SARS-CoV-2 Infection
Long COVID Series Part 4.2: An Introduction to the Development of Autoimmune Disease Post-COVID-19
Introduction
Autoimmune disease ‘self-immune disease’ (auto is the Greek prefix for ‘self’) refers to a set of over 80 different diseases that have one thing in common: The immune system has mistaken ‘self’ proteins, proteins our own body produces for a foreign invader.
The cells of the immune system are a powerful force that can rapidly eliminate pathogens like viruses, bacteria, and fungus that cause infections, but if they get confused and begin attacking a protein or set of proteins in our own body, debilitating disease can develop.
Some autoimmune diseases are incredibly rare, while others such as multiple sclerosis are well known. Others fail to adhere to a clear diagnosis, making both naming and sometimes treating them difficult. Autoimmune disease can affect every major organ system and often multiple organ systems at once as disease progresses. Below is compiled population based prevalence and average age of onset that uses data from the ~2010’s (reference link: here).
Autoimmune Disease, by the Numbers Author: Maddie Bender, Jen Christiansen, Miriam Quick Publication: Scientific American Publisher: SCIENTIFIC AMERICAN, a Division of Springer Nature America, Inc. Date: Sep 1, 2021 Copyright 2021 Scientific American, Inc.
As an immunologist who watched the trickle of data showing that the SARS-CoV-2 virus impacts the immune system turn into a flood, I have predicted that our data in 5 to 10 years from now will show a significant increase in diagnosed autoimmune diseases. Like cancer, autoimmune disease can take time to develop, even when it is triggered by a viral infection. Sometimes, however, autoimmune disease develops rapidly after infection.
So, can SARS-CoV-2 cause autoimmune diseases? And, does SARS-CoV-2 infection lead to elevated autoimmune disease risk relative to other viruses? I believe the answers are yes and yes, and below is the evidence to support these claims. First, let’s review some immune system basics that will help us orient to how this could be happening.
Please note that the papers referenced in this write up are not meant to be an exhaustive source but are representative articles of a large body of literature.
How Is Immune System Tolerance Developed and Maintained?
To understand how autoimmune disease develops it is important to understand how the immune system works. A core function of the immune system is maintenance of ‘tolerance’ or non-reactivity to self proteins. This can be thought of as making sure an army is loyal so they don’t start to attack themselves. The maintenance of self-tolerance is supported by multiple mechanisms that could fill two or more textbooks. Here, I have attempted to condense the topic and provide a general introduction to the concepts:
Central Tolerance: Early in the development process immune cells (each of which can recognize a unique protein signature) cells that react to your own body’s proteins are eliminated. For example, developing T cells are tested in the thymus. T cells that bind too strongly to self proteins are eliminated and do not enter circulation. B cells that exhibit strong self protein responses are subject to receptor (antibody) editing. When receptor editing fails to reduce autoreactivity (self reactivity) the B cells are arrested (shut down / becoming non-reactive) and may die off. This system is imperfect, and peripheral tolerance (after immune cells have matured) is critical to head off autoimmune disease development.
Peripheral Tolerance: Once T and B cells have matured and are released into circulation as naïve (antigen and activation inexperienced) cells, additional mechanisms are required to keep the cells of the immune system from attacking your own body. Some of these include tolerance maintenance by specific antigen presenting cell subsets. For example, regulatory T cells (Tregs) which make up between 5% to 10% of our T cell population suppress the activation of other T cells. If Tregs are lost, life-threatening systemic autoinflammatory disease quickly develops (imagine all autoimmune diseases showing up at once). Defects in genes necessary for Treg function lead to catastrophic systemic autoimmune disease and early death.
As for B cells, studies have reported that anywhere between 2.5% to 30% of B cells in circulation can produce autoreactive antibodies. Autoreactive B cells are kept from becoming activated by a variety of mechanisms, but their suppression isn’t complete. One cell type called Bregs is thought to play a role in reducing B cell autoreactivity. Not as much is known about Bregs as Tregs, but this population of B cells is thought to also help keep the B cell population from producing autoantibodies. Also, it is important to note that in perfectly healthy people autoreactive antibodies can often be found in their blood. It has been hypothesized that in some cases these antibodies may be helpful.
Autoreactive Antibodies: Having circulating autoreactive antibodies does not always indicate disease, but all people with autoimmune disease that has B cell participation have autoreactive antibodies in circulation. In some cases, autoreactive antibodies can be a predictor of autoimmune disease years before it develops. One example is the appearance of anti-α-enolase antibodies, and anti-mutated citrullinated vimentin antibodies which show up in serum years before development of rheumatoid arthritis.
In summary, the immune system works hard to keep it’s own power in check. Therefore, most people are able to fight off pathogens while maintaining tolerance to their own proteins. However if this system is pushed too far for too long by inflammation or environmental factors, tolerance to ‘self’ can be broken down. Also autoimmune disease is often considered idiopathic, meaning there was no clear cause of the disease. However, we do know that viral infection can trigger autoimmune disease and genetics can increase the risk of developing autoimmune disease. Also there is a hormonal component to autoimmune disease and X chromosome driven component as well where the vast majority of autoimmune diseases skew towards being more prevalent in those with estrogen dominant hormone profiles or a double X chromosome.
How Do Viruses Trigger Autoimmune Disease?
The threshold for how far out of balance an immune system can be pushed before tolerance breaks down and autoimmune disease is developed is different for each person. Currently, the risk of autoimmune disease is predicted by a complex map of a person’s genetics, age, hormones, diet, smoking, environment, and viral infection.
It is well known that both acute (short term) and chronic (long term) viral infection can disrupt and alter immune system function.
The immediate impact of viral infection is often a high fever, aches, and generally feeling miserable for three or so days to a couple of weeks in the case of some SARS-CoV-2 infections. During this acute phase of infection, the cells of your immune system are releasing high amounts of cytokines and effector molecules into your blood stream, activating cells in an ‘all hands on deck’ manner and ‘we’ll clean up later’ frantic state of emergency. This can lead to activation of cells that would otherwise not be activated in what is called a ‘bystander’ effect. (Pacheco, Y. 2019 et al; Fujinami, R.S. et al.; Getts, D.R. et al.).
This is why many immunologists are especially concerned about the extended periods of extreme acute inflammation seen in patients with COVID-19 and the worrisome early reports of Tregs disappearing. On the other hand, higher exhaustion marker expression by T cells for months post-COVID-19 may play a role in reducing autoreactivity. A similar pattern can be seen in a highly pathogenic influenza infection and in an influenza infection in older patients; however, it still does not appear to be as severe as T cell exhaustion post-COVID-19.
After the immune system has controlled a viral infection, some viruses are not completely cleared (removed) from the body, they continue to hide from the immune system. This is a chronic viral infection. Many viruses can take up long-lived (sometimes life-long) residence in different tissues in our bodies. These viruses may lay dormant, escaping immune system detection, and they may occasionally be found again by the immune system leading to an inflammatory response. One common example of this is the development of a shingles rash. This is the re-emergence of the varicella zoster virus (the virus that causes chicken pox) which often occurs when the immune system is weakened. Sometimes long-lived viral infection can cause cancer, as is the case with HPV, in other cases they may trigger autoimmune diseases including multiple sclerosis and other demyelinating diseases.
If you can imagine, the immune system is always working to find and battle the invaders. With prolonged infection, over the course of years, it’s like playing a game of whack-a-mole where the virus can go into hiding before re-emerging months or years later. Sometimes the immune system will miss and occasionally self-proteins may be mistaken for the virus (epitope spread), particularly if the virus has proteins that look similar to your own native proteins (molecular mimicry).
Indeed, during both the acute (immediate) and chronic (years long) phases of infection viruses that produce proteins that look similar to your own (molecular mimicry) can activate potentially autoreactive cells that would otherwise be dormant.
In summary, several viruses have been linked to the development of common autoimmune diseases, below is a table with some examples. (Please see reference here for the full table).
Now that we’ve reviewed the concepts of immune tolerance, how viruses might break tolerance, and how many viruses may trigger the development of autoimmune disease, let’s get back to the SARS-CoV-2 virus. The SARS-CoV-2 virus has been shown to have both an acute and a chronic phase where virus can continue to live in tissues for months, possibly years. So, can SARS-CoV-2 trigger autoimmune disease?
Evidence That SARS-CoV-2 Triggers Autoimmune Disease
The SARS-CoV-2 virus, which causes the disease COVID-19 can have a long, highly inflammatory acute phase as well as a chronic phase, taking up long-lived residence in tissues. In March 2024, UC San Francisco scientists published that they found the SARS-CoV-2 virus in blood 14 months after infection and in tissue samples up to two years after infection.
Already, SARS-CoV-2 infection has been demonstrated to be a risk factor for a growing list of autoimmune diseases. Below are the results of 3 peer reviewed publications that collectively studied over 2 million people and found an elevated risk for a variety of autoimmune diseases post-COVID-19.
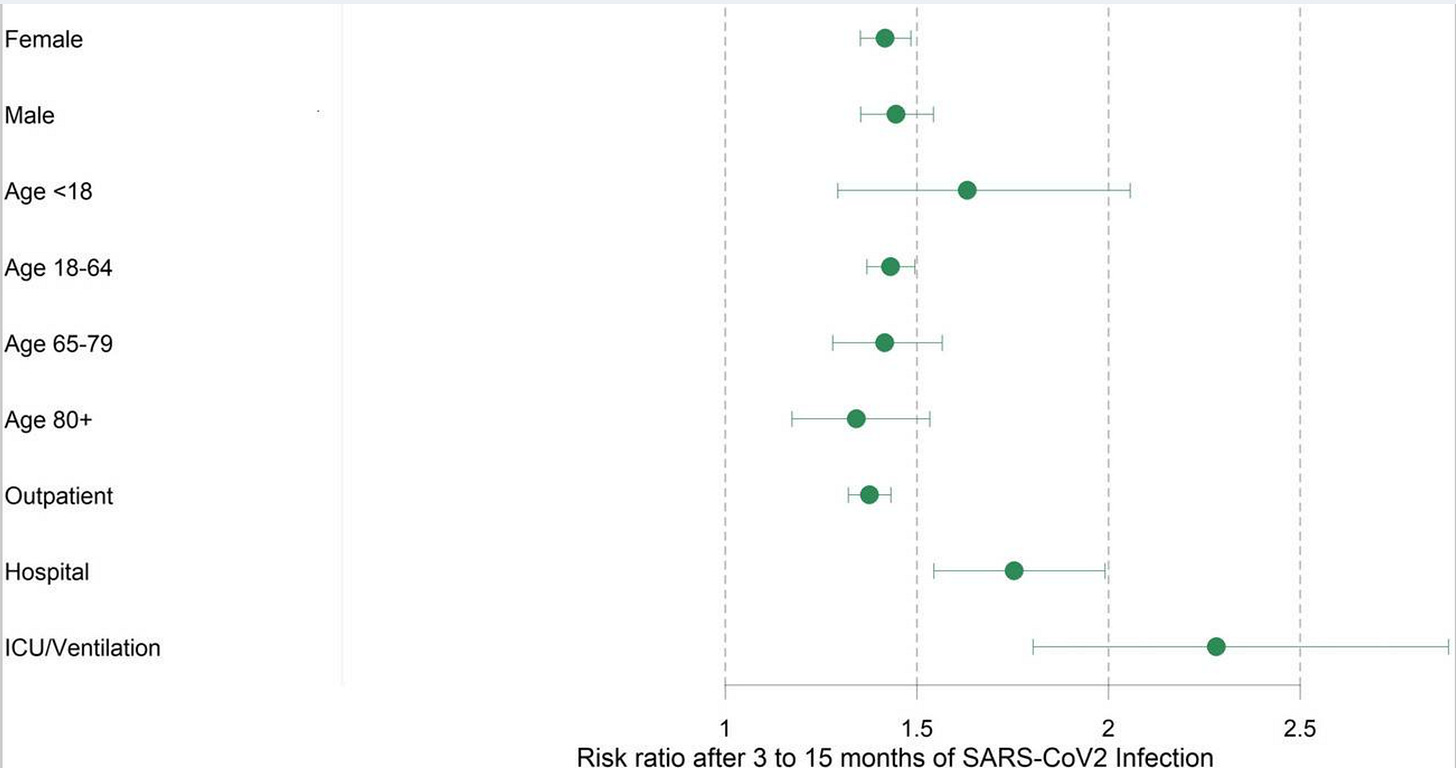
For example, Tesch, F. et al. found that in a group of 641,407 people that risk of developing first onset autoimmune disease was 37% to 48% higher within the first 3 to 15 months after infection compared when compared to 1.9 million uninfected controls. In their study they found that severity of disease was positively correlated with an increased risk of developing autoimmune disease (figure below).
Interestingly, the diseases with elevated risk not only included several common autoimmune diseases, but many rare autoimmune diseases often associated with vascular inflammation. As discussed above if a virus is causing chronic inflammation in a particular tissue the immune system may begin to make mistakes and start attacking the tissue itself, and SARS-CoV-2 infects the cells that line our blood vessels. Here is an excerpt of the findings from the study:
“The most common incident autoimmune diseases with an IR above 1 in 1000 person-years in the COVID-19 group were Hashimoto thyroiditis (IR COVID-19 =4.41, IRR=1.42, 95% CI=1.33–1.52), Graves’ disease (IR COVID-19=3.52, IRR=1.41, 95% CI=1.31–1.51), psoriasis (IR COVID-19=3.17, IRR=1.17, 95% CI=1.09–1.26), rheumatoid arthritis (IR COVID-19 =2.43, IRR=1.42, 95% CI=1.30–1.56), and Sjögren syndrome (IR COVID-19=1.24, IRR=1.44, 95% CI=1.27–1.63). In addition, the more specific disease definitions with medication led to similar results (Table 2). The highest IRRs, i.e., the largest significant effect estimates, were found for Wegner’s disease (IRR=2.51, 95% CI=1.42–4.46), Behcet’s disease (IRR=2.42, 95% CI=1.10–5.35), sarcoidosis (IRR=2.14, 95% CI=1.73–2.65), and arteritis temporalis (IRR=1.63, 95% CI=1.05–2.53). Wegner’s disease, Behcet’s disease, and arteritis temporalis belong to the group of rare autoimmune diseases involving vasculitis, i.e., small vessel inflammatory processes.”
Another large cohort study (Chang, R. et al.) compared the hospital records of 887,455 patients diagnosed with COVID-19 to 887,455 people who tested negative for SARS-CoV-2. In this study the researchers also found a significantly elevated risk for the development of several autoimmune diseases within 6 months of infection and an increased risk of mortality.
Per the study: “The Kaplan–Meier curves of all the autoimmune disease outcomes also indicated difference of probability between the two cohorts (Log–Rank test, p < 0.001, Fig. 3).”
Interestingly, the researchers did not find a significant difference in the risk for autoimmune disease development between men and women. This is surprising as autoimmune disease typically impacts women at a higher rate (figure below).
Finally, in Peng, K. et al. a retrospective study of 1,028,721 COVID-19 recovered individuals and 3,168,467 non-COVID-19 controls, an elevated risk of developing several autoimmune diseases was identified. Higher risk ratios were identified in this study for: Immune mediated thrombocytopenia, Psoriasis, Rheumatoid arthritis, Anti-phospholipid antibody syndrome, Pemphigoid, Pernicious anaemia, Spondyloarthritis, Multiple Sclerosis, Graves’ Disease, and Vasculitis.
Is The Risk Of Developing Autoimmune Disease Higher After SARS-CoV-2 Infection Relative To Other Viruses?
Having another virus in the milieu of common infections that can trigger autoimmune disease is at best additive to the risk of autoimmune disease development. At worst it may hasten and increase the magnitude of inflammatory events, exacerbate ongoing subclinical inflammation, or upon immune system exhaustion enable re-emergence of latent viruses such as the varicella-zoster virus which causes Shingles. (Yes, there is a higher rate of incidence of shingles development post SARS-CoV-2 infection, a review and call to action can be found here).
But, how much more risky is it to get SARS-CoV-2? It is currently unclear what the answer is to this question, but early studies do indicate that SARS-CoV-2 infection is associated with higher risk of autoimmune disease than another respiratory virus, influenza. Many of these studies look at the development of autoantibodies which is sometimes an early indicator of autoimmune disease development. Here, I have compiled information from an example set of studies comparing the two, the first one looks at infants:
In Lugar, M. et al. 885 infants genetically predisposed to development of Type 1 Diabetes were studied to determine if COVID-19 or influenza exposure increased the risk of developing type 1 diabetes. Researchers found that the development of islet autoantibodies was associated with SARS-CoV-2 antibodies (indicating infection or exposure) but not H1N1 influenza antibodies. Islets of Langerhans are the part of our pancreas that produces insulin and is attacked by the immune system in type 1 diabetes.
In Kim, M.S. et al. researchers looked at the health records of 10,027,506 Korean and 12,218,680 Japanese and matched patients with COVID-19 to patients with confirmed influenza infection, and uninfected controls. In comparing a total of 94,504 patients in Korea that had either COVID-19 or influenza and 110,310 (COVID-19) to 115,003 (Influenza) in Japan the team found an increased risk for incident autoimmune rheumatic disease (AIRD) for up to 12 months after COVID-19 diagnosis compared with influenza-infected and uninfected control patients. They also noted that the greater the severity of the SARS-CoV-2 infection the higher the risk of autoimmune disease development and that vaccination reduced the risk of autoimmune disease development (except in cases of vaccine breakthrough and development of severe disease). Note: Autoimmune rheumatic disease (AIRD) refers to a group of multisystem inflammatory autoimmune diseases which include: Rheumatoid arthritis, Systemic lupus erythematosus, Psoriatic arthritis chronic inflammatory arthritis, sarcoidosis, systemic vasculitis and other connective tissue diseases.
In Wang, E.Y. et al. researchers demonstrated a significant increase in the amount and diversity of autoantibodies with mild or asymptomatic SARS-CoV-2 infection. Many of these antibodies contributed to worse COVID-19 disease in animal models. These antibodies were not found in patient controls, indicating that even subclinical infection can lead to potentially worse outcomes and the development of anti-tissue antibodies found in adults presumably previously infected with typical respiratory pathogens. The authors conclude: “The extent of autoantibody reactivities seen in patients with COVID-19 suggests that humoral immunopathology is an intrinsic aspect of the pathogenesis of COVID-19.”
Finally, in Feng, A. et al. researchers looked at autoantibody development during a myriad of infections including bacterial and fungal infections, as well as influenza and Marburg virus. This team of researchers found that indeed many infections trigger the development of autoantibodies. Unlike other studies they did not see a statistically significant difference in the handful of antibodies they examined between COVID-19 patients and Influenza patients, however they did show a trend towards more COVID-19 people developing autoantibodies. Due to the small size of the patient cohort (n =19 COVID-19 recovered patients v. 25 influenza recovered patients) and in the context of other published data it is likely that this trend would show statically significance in a larger population. Additionally, even if autoantibody development turns out to be similar, the average adult is infected by influenza once every 5 to 7 years while SARS-CoV-2 infection is far more common.
The counter argument regarding SARS-CoV-2 infection being harmful has been a bit of a handwaving idea that it provides protective immunity. While this is true to a point, the numerous virus variants that have accumulated have enabled SARS-CoV-2 to quickly evade prior immune responses and infect someone with a different variant. Re-infection with a different variant has now been documented as early as 2-3 weeks after initial infection. In addition, immunosuppression, poor outcomes with second and third infections, continued rapid virus mutation, and the fact that this virus can take up long-lived tissue residence leaves most people susceptible to repeat infections and long-term chronic infection.
Summary
Unfortunately, once triggered, autoimmune disease does not have a cure. Fortunately, clinical management of autoimmune disease has evolved significantly in the last two decades. Targeted therapeutics with fewer side effects have been developed and are now used as first line therapeutics. However, while the immune system continues to ‘hunt’ for the bad guy, even subclinical (no symptoms) inflammation can lead to ‘epitope spread’. This can increase the speed of tissue damage and the frequency of flare ups. Viral persistence in tissues can hasten this process by continuing to trigger immune responses. Therapeutic failure (when the drug no longer controls disease) is common among autoimmune disease drugs. The good news is that clinicians do have quite a few tools at their disposal to help manage autoimmune disease progression, and can often successfully switch a patient to a new drug. But, the best way to treat a disease or infection is prevention.
Prevention of infection is still the best way to prevent infection associated disease. However, vaccination and taking Paxlovid (which disrupts virus replication) early likely reduce the chance of developing virus-triggered autoimmune disease as they can significantly reduce disease severity. However, this is not a guarantee. Which is why I personally continue to wear a high quality mask (N95, KN95, KF94 that is NIOSH approved and makes a good seal around my face and nose) as part of my prevention measures. Even asymptomatic SARS-CoV-2 infection has been linked to development of long COVID.
Currently, there is sufficient evidence to link SARS-CoV-2 infection to a higher risk of developing autoimmune disease. However, the risk appears to be variable between autoimmune diseases and therefore it is difficult to define an absolute risk for each of the 80 or so characterized autoimmune diseases. It is interesting that COVID-19 appears to increase the risk of developing some autoimmune diseases such as rare vascular associated autoimmune diseases. Also, it is surprising to see that in a large cohort study both men and women had an equal increase in the risk of developing certain autoimmune diseases post-COVID-19. Immunologists have long understood that viral infection can trigger autoimmune disease, and respiratory viruses like influenza may trigger some autoimmune diseases, but current evidence indicates that SARS-CoV-2 infection elevates the risk of developing autoimmune disease far more than any other respiratory virus.
Subscribe to join me for Part 4.3 on Immune System Dysfunction and Dysregulation where I will discuss other diseases that are linked to weakened immune response post COVID-19 and emerging evidence that may support a link to increased risk of cancer development.










Such an interesting read. I was struck by the timeframe the studies were saying risk was highest - with many being within 12 months, it makes me wonder if Covid caused the autoimmune disease *or* triggered or reactivated it? It’s interesting to me because in Feb 2020 I got a viral infection that caused long term symptoms that ended up leading to a Lyme disease diagnosis that was likely from an infection years ago I didn’t even know about!
I greatly appreciate your work and hope one day there will be a post about how we have strong therapeutics and/or vaccines that can reduce the damage this virus does!