Does Paxlovid Really Work?
A new study calls into questions the efficacy of Paxlovid. Here I review the study and why I would choose to take Paxlovid if infected.
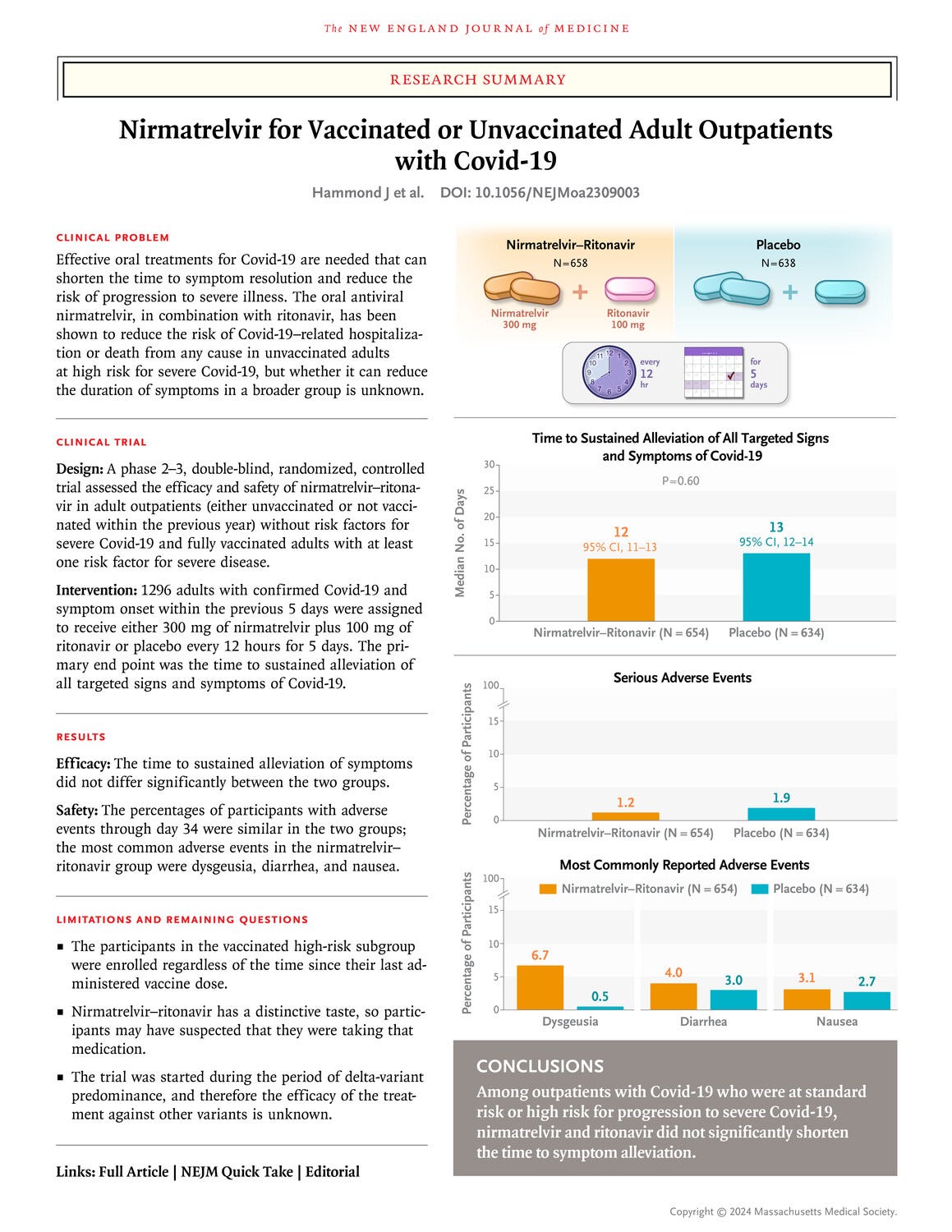
Published on April 3rd, 2024 in The New England Journal of Medicine “Nirmatrelvir for Vaccinated or Unvaccinated Adult Outpatients with Covid-19” claims there is no benefit to taking Paxlovid for COVID-19. The New England Journal of Medicine is a highly regarded journal so I paused to read the article in full and was disappointed by how the data was analyzed, what data was presented, and I was left with more questions than answers in light of other larger studies that clearly demonstrate the efficacy of Paxlovid.
The corresponding author is employed by Pfizer, the company that makes and distributes Paxlovid. While it is nice to see a pharmaceutical company publishing results that don’t agree with their bottom line, the study was lacking due to size and subgroup analysis. The wide range of patients, failure of included subjects to complete the full dosing course of Paxlovid, along with the small study size are likely why the study failed to find statistical significance. This is a case of absence of evidence is not evidence of absence.
First it should be addressed that a higher rate of severe adverse events was reported in the placebo control group. Non-severe adverse events were not statistically different between the two groups. It is important to note that regardless of Paxlovid being helpful or not, it did not cause harm to those taking the medication.
Overall results showed reduction in hospital stays and lower rates of hospitalization leaning towards Paxlovid being beneficial, but the results were not statistically significant. For example, five (5) people in the Paxlovid group were hospitalized due to COVID-19 infection or died from any cause while ten (10) in the placebo group were hospitalized due to COVID-19 or died from any cause.
Given the data that was collected during this Phase II/III trial here are some of the areas where I believe the analysis fell short or may skew the results.
Vaccination Status and Serology: Both unvaccinated and not vaccinated in the previous 12 months were both considered ‘unvaccinated.’ Given the extensive life-time of memory T and B cells, often for decades, never vaccinated will not generally have the same outcome as vaccinated 12 months or more prior. Indeed, clinical data bears this out wherein never vaccinated still die and are hospitalized at higher rates that those with two vaccines. Though this gap is closing, it is still important to note that memory immune cells reduce disease severity. Therefore it would be important to understand how clinical progression of COVID-19 is impacted by Paxlovid in a truly unvaccinated cohort rather than combining the groups, which at this point may include people that have had as many as three boosters but still may not have been vaccinated in the last 12 months. The authors say in the discussion that this is acceptable due to mutations in the virus; however, SARS-CoV-2 mutations do not impact T cell recognition of the virus and memory B cells have been shown to quickly adapt to new variants. Sars-CoV-2 mutations impact initial infection based on circulating antibodies but do not have as strong of an impact on memory immune responses which reduce hospitalization and death.
Recently vaccinated was not well defined. It is unclear which vaccine was taken and what it’s likelihood of matching the current variant in circulation was during the time of the study, this too may impact severity of disease. Also, how many vaccines the patient had been given in the prior 12 month period was not disclosed. Several high-risk individuals have been approved for vaccination on a roughly 6 month interval and recent vaccination may alter results.
People who had been prior infected with COVID but not vaccinated were not separated in the analysis. In both treated and untreated groups 73% of participants were seropositive. However, the study considered people who had antibodies to either the nucleocapsid protein or the spike protein to be seropositive. Therefore being seropositive in this study does not distinguish between a participant being vaccinated, unvaccinated but exposed to the virus, or having hybrid immunity. It would have been of interest to understand the difference between COVID-19 naïve unvaccinated and vaccinated populations. Based on other publications these differences would be expected to result in different clinical outcomes.
Significant comorbidities were not separated out in analysis: In the participant groups BMI ranged from 14 to 58.2 , both ends of this BMI spectrum would be potentially considered high-risk of severe infection. Median BMI was identical across all groups; however, the median may not account for actual group distribution which can significantly skew results one way or another. In the reported results in the main publication it is not clear that BMI was considered as a risk factor though this is well known to contribute to poor COVID-19 outcomes. It is stated that most participants had a BMI of 25 or higher which is generally considered overweight. Additionally, the Paxlovid group had slightly more participants that were above the age of 65, and a few more in the treated group also suffered from Hypertension and Diabetes. In the placebo group more had a high BMI. Although not reported as a risk factor, there were more men in the Paxlovid group and it is widely reported that men have more severe COVID-19 disease. Together these risk factors were skewed higher in the Paxlovid treated group. In such a small patient population tens of patients being higher risk may obscure the impact of a therapeutic such as Paxlovid.
Paxlovid Adherence: Adherence was measured as taking at least 80% of the 5 day course of medication indicating that a full day missed was interpreted as a full dose set or more as adherence was also described as going up to 115% meaning additional tablets were taken. It is unclear in the treated groups what percentage of people took the full dose, less than the full dose, or more.
Low number of trial participants: Though there was double the number of deaths and hospitalizations in the placebo group, the improvement was not statistically significant. This is in contrast to slightly larger real-world studies (example data table below). Additionally, 3 of the 10 in the placebo group were admitted to the ICU and none of the Paxlovid recipients were admitted to the ICU. Again, the numbers in each group were too low to determine statistical significance.
Summary: What this trial teaches us is that it is increasingly difficult to run conclusive studies testing COVID-19 therapeutics. Given the wide array of individual responses, range of co-morbidities, vaccination, and exposure statuses large groups are necessary to determine statistical significance. Larger (n = 180,000 to 1M+) real-world studies have demonstrated clear and significant benefit in taking Paxlovid early and are generally in agreement. References are linked here, here, and here. One study in particular showed a reduction in multiple Long COVID outcomes regardless of vaccination or boosted status. Given the lack of statistical significance and the small study group as well as a failure to categorize vaccination within the prior 12 months as being protective I am not surprised there was no statistical significance. If I personally were to acquire SARS-CoV-2 this smaller study would not discourage me from taking Paxlovid in light of several larger studies that clearly demonstrate overall benefit.




Thanks for this. I was a bit perplexed at first glance at the data. I made a short note of it in a reply in someone's note. Thanks for fleshing it out more. The one thing that struck me was the age ranging from 18-87 with just 5% over 65 and a median of 42. Symptoms alleviation in 12 vs 13 days means that we are talking about the second of the bi-phasic, the cytokine inflammatory, phase. Not the viral replication phase. Imagine my naive thinking that reducing hospitalization, ICU and death was the goal.
My immunologist , (I have PID) did not recommend paxlovid - in fact the academic medical center he is part of, does not use paxlovid . Perplexed , but trusting my docs. Thanks for explaining the science behind paxlovid!