COVID-19 Inflammatory Responses Acute and Chronic
Long COVID Series Part 2: Viral infection causes inflammation, but what exactly is inflammation and why is it a major contributor to Long COVID?
The US FDA has not yet approved any drugs with the indication to prevent or treat Long COVID. Several clinical trials are ongoing and some are currently enrolling. I hope that this series provides helpful education to those suffering from or with loved ones suffering from Long COVID.
Inflammation is a normal part of the body’s response to infection and injury. Inflammation can be localized (at the sight of a bruise for example), specific to a particular tissue (hives on the skin), or it can impact the entire body.
Image from Medpage Today: Olfactory Axon Injuries, Microvascular Pathology Seen in COVID-19
During acute (temporary) inflammation and proteins that trigger inflammation are part of how the immune system communicates that there is a problem, and how immune cells are directed to tissues where a virus or bacteria has entered the body. Even non-immune system cells release inflammatory signals when damaged communicating to the immune system that something has gone wrong. Pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α can cause fever, inflammation, and are involved in inducing pain. Also, they can directly activate cells of the immune system, inducing the cells to mature and fight off pathogens. Unfortunately when immune cells fight off pathogens they often eliminate cells and tissues that are virally infected. Because SARS-CoV-2 can enter any cell bearing an ACE2 receptor and it is ubiquitously (all over the body) expressed this means that skin cells, cells lining blood vessel walls, neurons and more may be targeted by our own immune system.
Cytokine release can also lead to physiologic changes beyond fever. Vascular leak and damage, cardiac arrythmias, and in extreme cases of Cytokine Release Syndrome (CRS) cognitive impairment, respiratory distress, circulatory shock and organ failure.
Severe viral, bacterial, fungal, or parasitic infection can cause a feedback-loop of cytokine release leading to CRS. Although the SARS-CoV-2 virus does have some cytokine suppressive capabilities, CRS is a major cause of morbidity in SARS-CoV-2 infected patients. COVID-19 can cause acute respiratory distress syndrome (ARDS) which is a systemic inflammatory condition, rather than a local pulmonary process. Notably, not all approaches to inflammatory suppression work well for management of cytokine storm. Sars-CoV-2 induces significant release of cytokines, including but not limited to L-1, IL-4, IL-6, IL-7, IL-10, IL-12, IL-17, and IL-18), IFN-γ, TNF-α and TGF-β. Many of these cytokines bridge distinct branches of immune responses and both the magnitude of cytokine release as well as the broad nature of it may be involved in triggering autoimmune disease and aberrant inflammation.
Per the CDC (graphic below) clinical symptoms post COVID-19 infection include the following non-exhaustive list:
Many of these symptoms may be linked to changes in the immune system leading to chronic inflammation and, or immune system activation.
Chronic Inflammation in Long COVID
Chronic inflammation after Sars-CoV-2 infection is a well-documented symptom of Long COVID. Continued inflammation may be part of a healing process within damaged tissue, wherein the symptoms would be expected to slowly improve with time as is seen in some people re-gaining their sense of taste and smell. However, some inflammation appears to persist and cause many of the symptoms of Long COVID. Based on current research literature on chronic inflammation in Long COVID, I believe it will be divided into three categories; 1) autoimmune driven inflammation, 2) persistent idiopathic inflammation, and 3) chronic viral infection induced inflammation. Chronic viral infection or persistent inflammation may be a driver of autoimmune related inflammation or development of autoimmune diseases. Currently we do not have enough studies to link either of these outcomes to persistent viral infection, however in other instances of chronic viral infection autoimmune disease may be triggered as in the case of Epstein–Barr virus and multiple sclerosis. Below I will make the case for each based on current literature and share just some of the ways treatment has been approached. As always none of this research is intended to diagnose or serve as medical advice, but as an educational piece hopefully a helpful starting point for a conversation with a primary health care provider.
Autoimmune Disease and Long COVID
Given the enormity of this topic I will be writing about autoimmune diseases linked to SARS-CoV-2 infection in upcoming newsletters. However, it is important to acknowledge that viral infections can trigger the development of autoimmune disease. Although research is still being done in this area, studies have demonstrated that severe infection with both influenza and SARS-CoV-2 can induce development of autoantibodies. With SARS-CoV-2 infection antibodies against a variety of tissues are developed the number of which increase with disease severity (I have not found a comparable study examining a different virus). Although autoantibodies do not necessarily indicate autoimmune disease, those that do have autoimmune disease have autoantibodies in circulation. Sars-CoV-2 infection is linked to development of Type I Diabetes, and several case reports as well as temporal examination of COVID-19 and Graves Disease indicates that they may be linked. Two studies summarized here each examined cohorts of over 600,000 SARS-CoV-2 infected people and found an increased incidence of autoimmune disease post COVID-19. If you are suffering from Long-COVID it may be a good idea to discuss ruling out new onset autoimmune disease with your primary care provider. Once autoimmune disease is developed it is life-long, however catching it early and starting immune modulating therapeutics can slow disease progression.
Persistent Idiopathic Inflammation in Long COVID
Immune system dysfunction can lead to physical symptoms that are not driven by chronic viral infection or autoimmune disease. Ongoing issues post viral infection that can not be attributed to acute damage caused by viral infection or autoimmune driven diseases will be difficult to categorize as ruling out autoimmune diseases is critical. For the sake of this short post I am summing up inflammation without an identified root cause post COVID-19 infection as idiopathic and assuming it is not autoimmune in nature or triggered by chronic viral infection. One example of inflammation that seems to be unrelated to autoimmune disease or chronic viral infection is the development of idiopathic hives in people that have not prior experienced hives, also known as urticaria. Though the literature is sparse with only a handful of case reports (examples are linked here, here, here, and here) indicate that development of hives post COVID-19 may be controlled with over-the-counter anti-histamines, but in some cases reported in the literature second and third-line therapeutics were needed. Viral infections, even the common cold, can trigger development of hives, or worsen pre-existing conditions, often occurring a few weeks after infection. As always one should always consult their primary health care provider and discuss any new symptoms after viral infection.
There are many approaches your physician may take to address persistent inflammation. Some small research studies have indicated that corticosteroid treatment may be beneficial for Long COVID and can be found here, here, and here. In searching ClinicalTrials.gov only one trial (NCT05648734) investigating the use of corticosteroids in Long COVID was found, though completed the study has not posted or published results. Several Clinical Trials are being initiated via the US National Institutes of Health (NIH) RECOVER initiative, though I have not seen one investigating corticosteroid use on their site though the immunological aspect of Long COVID is acknowledged.
In addition to corticosteroid therapy a recently reported biomarker for Long COVID reported in Science in January, 2024 could be a more targeted way to address chronic inflammation in Long COVID.
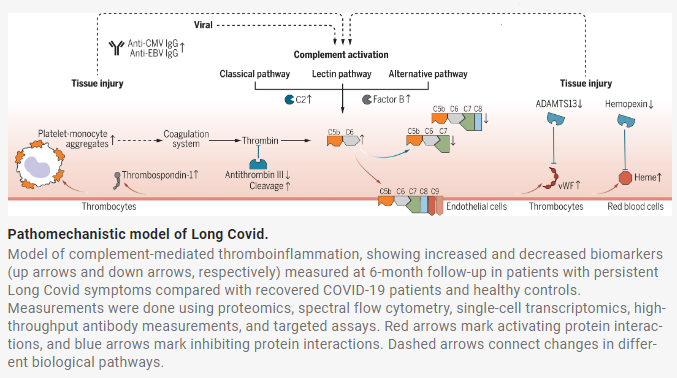
In the study researchers were able to identify elevated complement protein levels in a longitudinal study of 113 Long COVID sufferers over the course one year. In examination of over 6,500 serum proteins an increase in complement complex C5bC6 was identified as significantly higher in study participants with persistent Long COVID.
Complement proteins are part of the immune systems non-specific inflammatory response and can cause tissue damage when activated. The cleavage products of complement C5 are proteins C5a and C5b wherein C5b links up with C6 to continue a cascade of inflammatory activation while C5a itself is highly inflammatory capable of driving histamine release and neutrophil activation. Immunologists have studied the 3 pathways of complement activation for decades and C3 followed by C5 are two points of convergence for the Classical, Lectin, and Alternative pathways. Eculizumab (and at least 3 biosimilars) is an FDA approved antibody that blocks C5 cleavage into C5a and C5b and is approved to treat patients suffering from paroxysmal nocturnal hemoglobinuria (PNH). If the study published in Science bears out that C5bC6 complexes are a consistent biomarker of Long COVID, investigation into C5 cleavage blockade may be fruitful for reduction of inflammation and tissue damage associated with Long COVID.
Indeed a small open-label study showed modest improvement using C5 inhibitor Zilucoplan in hospitalized COVID patients, though not not statistically significant for a walk test or 28 day mortality (9% in treated, 21% in control) this may mostly be due to the small number of patients in each group (n = 55 treated v n = 26 untreated) and warrants further investigation. The study reported high tolerance and no safety issues.
C5 inhibition also typically elicits a significant decline in C-reactive protein and IL-6 levels during active infection and blockade of C3 (upstream of C5 in the complement cascade) has also been shown to reduce inflammation during active severe COVID-19. A study looking at inflammatory markers IL-6 and C-reactive protein found that both were elevated in those suffering from Long COVID relative to controls. Another study indicated that baseline IL-6 can be used to predict development of Long COVID in those hospitalized with COVID infection.
A quick search of ClinicalTrials.gov did not show any ongoing, registered, or completed trials of C3 or C5 inhibitors to treat Long-COVID. Notably chronic inflammation may be linked to chronic fatigue, another prominent symptom reported in Long COVID, although this research is ongoing several pre-SARS-CoV-2 studies have linked chronic inflammation to chronic fatigue. (References can be found here, here, here, and here)
Due to it’s identification as a biomarker, investigating the role of the complement pathway activation in Long COVID may be helpful for those suffering from Long-COVID.
Chronic Viral Infection
Viral reservoirs are a prime suspect for Long COVID, however it is difficult to test for active viral residence in tissues in living people. This requires biopsy. However, multiple research studies have demonstrated evidence of continued Sars-CoV-2 replication after primary symptoms have ameliorated, including continued shedding in stool and virus in brain and other tissues found during post-mortem biopsy. Here are select references linking Long COVID to viral persistence:
Association of SARS-CoV-2 infection and persistence with long COVID Chengliang, Y. et al. The Lancet Respiratory Medicine, 2023
Serotonin reduction in post-acute sequelae of viral infection Wong, A. C. et al. Cell 2023
Prevalence of persistent SARS-CoV-2 in a large community surveillance study Ghafari, M. et al. Nature, 2024
Several small research studies and case reports (linked here and here) have indicated that Paxlovid which stops viral replication is useful for treating Long COVID though in some studies results indicated it may be helpful for some people but not others. Notably the NIH RECOVER-VITAL trial is investigating the use of Paxlovid to possibly eliminate persistent virus that is currently enrolling by invitation.
There are anecdotal reports [and case studies?] of vaccine boosters leading to rapid alleviation of Long COVID symptoms. This supports the hypothesis that chronic viral infection is a root cause of Long COVID in some people. An important piece of data that I feel is missing from these studies is the immune status of these individuals and if age and perhaps being immunocompromised plays a role in supporting continued viral infection.
Anecdotally, some people have reported no impact of vaccination on their Long COVID symptoms. It is unclear if this is due to latent viral infection driving Long COVID pathology in some people but not others, differences in immune response, distinct yet-to-be-defined categories of Long COVID, or a combination of all three.
Summary
Chronic inflammation post SARS-CoV-2 infection may be triggered by a variety of factors that can work in concert. Damage to the body and immune system dysfunction may result from both acute infection as may persist in response to chronic viral infection or as a manifestation of autoimmune disease or immune system dysregulation leading to chronic inflammation that is not tissue specific (as is the case with most autoimmune disease). Several clinical trials are ongoing, however I was unable to identify any trials examining the use of corticosteroids or complement system targeting therapeutics for which there is small research study support that these lines of investigation may be fruitful. Today we have a far better understanding of Long COVID and thanks to research in the autoimmune and inflammatory diseases there may be already approved therapeutics that will have a significant impact on Long COVID management.





Do you believe we will have better therapeutics to manage long Covid in the near future?