The Metabolic Impact of Long COVID
Long COVID Series Part 3: Emerging research shows that SARS-CoV-2 infection alters mitochondrial function
From chronic fatigue to brain fog, it’s clear that Long COVID has zapped the energy of millions of people. Some recover in a few months, some are still battling the symptoms years later. Here, the possible role of mitochondrial dysfunction in Long COVID is reviewed. I start by providing an overview of mitochondrial function followed by a reference list demonstrating how SARS-CoV-2 impacts mitochondrial function, and finally with a summary re: possible future treatments.
First, What is Cellular Metabolism?
The phrase ‘cellular metabolism’ elicits personal flashbacks to attempts to memorize the Krebs cycle while I was an undergraduate biochemistry major. It also a phrase that is widely abused across the ‘wellness’ industry promising to slow cellular metabolism down or speed it up in the name of treating anything and everything that ails people.
Cellular metabolism, which most often refers to function of the mitochondria, is critical for physiologic function. Mitochondria, as you may have heard, are the ‘powerhouse of the cell.’ These tiny organelles “little organs” produce the energy we need to think, move around, digest food, etc. Mitochondria are found in every cell our our bodies (except red blood cells). Mitochondrial dysfunction impacts a cell’s ability to thrive and carry out other activities, which can impact every aspect of human health. That’s every organ, every tissue, each cell in the body. It is impossible to discuss metabolic issues post-viral infection and not cover mitochondria.
Where Did Mitochondria Come From?
There is a strong hypothesis that millions of years ago, in the primordial soup of life, one lucky cell type engulfed a bacterial cell that was immune to the rising levels of toxic oxygen in the atmosphere. In fact, mitochondria happily process oxygen into water while creating cellular energy in the form of ATP. Together these two cell types created a wildly successful partnership that gave rise to all eukaryotic life forms. Simply put, mitochondria are the reason oxygen doesn’t kill us.
So What do Mitochondria Actually Do Otherwise?
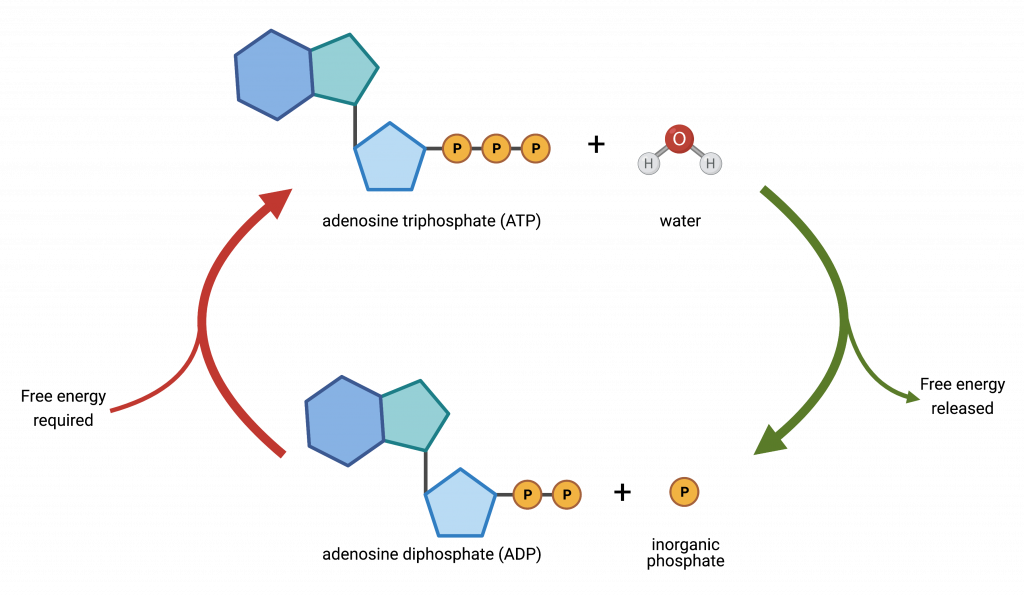
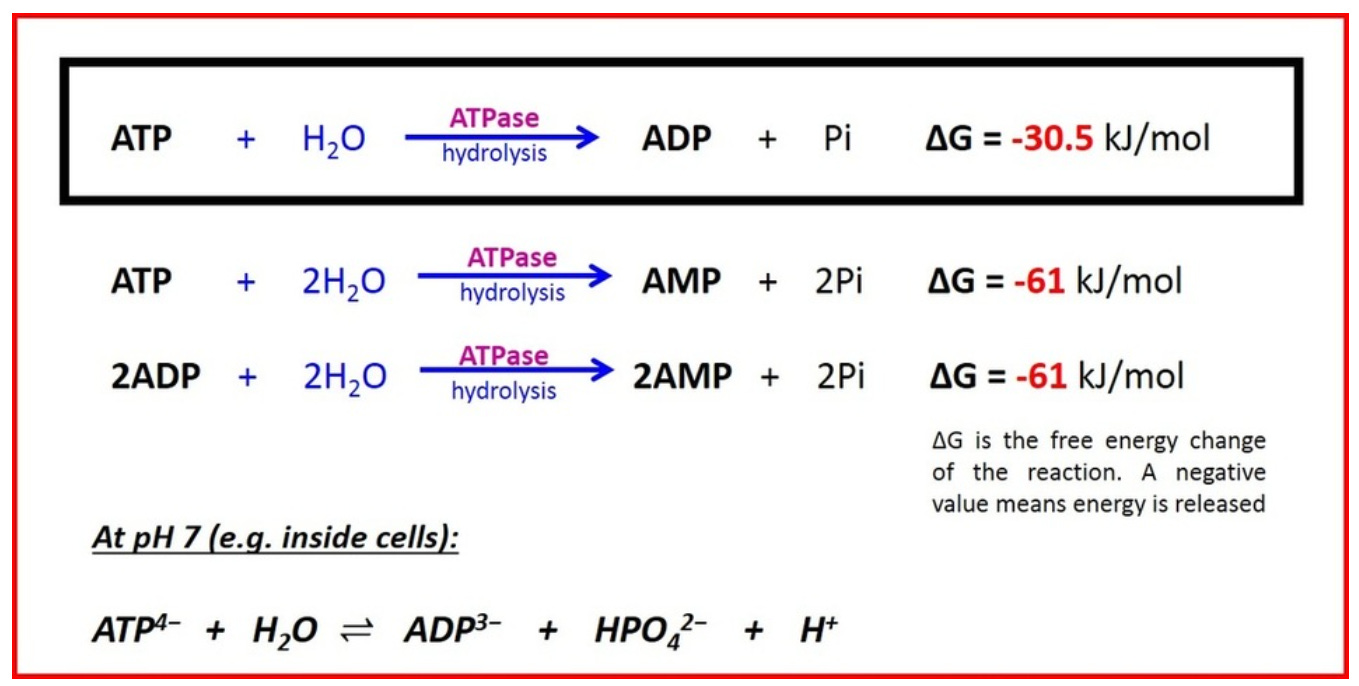
In cells mitochondria help with the end stage of converting glucose into stored chemical energy that can be rapidly used by cells. This chemical energy is stored in the molecule ‘ATP’ also known as adenosine triphosphate or ‘three phosphates.’ You can think of chemical energy as a type of stored up or spring loaded kinetic energy, it’s the book waiting to fall off the shelf and when it does energy is released. The energetic release in going from ATP to ADP (adenosine diphosphate, ‘two phosphates’) is 30.5 kJ/mol.
We’re getting into the realm of math that doesn’t sound real, but it’s probably a significant underestimate to say that a quadrillion ATP reactions occur every second in one person.
What’s important to remember is that the conversions between ATP and ADP or AMP (monophosphate) and back again as well as other reactions involving ATP support all eukaryotic life, including yours.
Adding that extra phosphate (P) is the job of the mitochondria and each human cell contains its own mitochondria, without which the cell would die. That’s not an exaggeration, the second the mitochondrial pathway shuts down it’s quickly all over, finito, adios, tot siens, do svidaniya; for a morbid example, this is how cyanide poisoning works, it disrupts the production of ATP.
What Do We Know About Viruses and Mitochondria Already?
When a virus invades a cell it can disrupt mitochondrial function. This damages the infected cell and if enough cells are infected in a given tissue, it will not function as well. Prolonged cellular stress caused by inflammation can also lead to loss of mitochondria in the cell and reduction that cells ability to function.
In other words, during viral infection mitochondria are slowing down their production of ATP leaving your cells, and possibly you, ‘huffing and puffing’.
What Happens When Mitochondria Don’t Work Well?
As one might imagine, diminished mitochondrial function is not good for you. Indeed, prolonged mitochondrial dysfunction is linked to the development of multiple diseases including neurodegenerative diseases, cancer, and cardiac issues, and chronic fatigue (representative publication linked, each of these fields is a very large area of research).
Does SARS-CoV-2 Impact Mitochondrial Function More Than A Typical Viral Infection?
I have not yet found a research report that directly compares mitochondrial dysfunction post-COVID to, for example, a bad case of influenza. However, there are several indications that SARS-CoV-2 may have a greater and more prolonged impact on mitochondrial function than an average viral infection.
The very high rate of post viral sequelae (Long COVID), wide range of Long COVID symptoms, and high number of Long COVID suffers reporting long-lasting fatigue and brain fog are a strong nod towards mitochondrial dysfunction. Notably, the brain is one of the most ‘energy hungry’ organs in the body with 100 to 1000 times more mitochondria in each neuron than the average human cell. If there is mitochondrial dysfunction the brain is at the top of the list for places it would first be noticed.
Determining if fatigue and brain fog are caused by a mitochondrial issue or are a result of tissue damage may not be possible. Damaged tissues often show mitochondrial dysfunction, and mitochondrial dysfunction can lead to poorly functioning tissues that accumulate damage that often looks like rapid aging. Most likely, it is a combination of both with high patient variability. In sum, we are left with a chicken and egg problem, but what is clear is that the SARS-CoV-2 is the causal agent.
How Does SARS-CoV-2 Impact Mitochondrial Function?
Upon digging into the literature, I started to feel like ‘how doesn’t it?’ I have found over 15 likely mechanisms (which are likely all contributors) for how SARS-CoV-2 likely impacts mitochondrial function, far to many to review here. But I will highlight some that are SARS-CoV-2 specific and have provided references for others below.
Some of the SARS-CoV-2 virus specific mechanisms rely on viral expression of the rarely discussed M and E proteins. One study showed that M protein can trigger mitochondrial death. Another demonstrated that the M and E proteins both disrupt calcium signaling within the mitochondria directly, disrupting it’s ability to maintain it’s membrane charge. This is bad news, especially for a mitochondria, that relies on charge differentials across it’s membranes to do it’s job producing cellular energy. These papers as well as a few others show that SARS-CoV-2 effectively hijacks the mitochondria for it’s own purposes while shutting down the mitochondria’s natural response to pathogens. These mechanisms are likely unique to coronaviruses, but were not well studied before the pandemic.
For those interested in deeper reading on the topic here is a sample of the numerous peer reviewed publications covering some of the ways that SARS-CoV-2 infection negatively impacts mitochondrial function:
I’d like to note that viral disruption of mitochondrial function is not a new concept. For a more in-depth description, an overview of general mechanisms by which viruses disrupt host mitochondrial function are reviewed here.
Future Directions
Today, it is not clear how many Long COVID patients can attribute their symptoms to mitochondrial dysfunction. However, given the persistence of mitochondrial dysfunction beyond 6 months in many cases and association with changes in skeletal muscle structure (as shown in this longitudinal case-controlled study, NCT05225688), it is likely a contributing if not causal of many Long COVID symptoms. In addition, genetic polymorphisms within mitochondria may explain why some are more predisposed to developing persistent Long COVID.
Treating Long COVID similarly to a mitochondrial disease may be an interesting treatment approach.
Mitochondrial diseases are typically rare, inherited genetic disorders for which there is not a cure. However, there are protocols for supportive care that may also be helpful for Long COVID sufferers as well. Several interventions used in supporting genetically inherited mitochondrial disease are reviewed here in a 2014 collaboration between researchers and physicians at the Cleveland Clinic and the Children’s National Medical Center in Washington DC. As always, talk with your doctor before attempting to treat anything as serious as Long COVID.
I remain hopeful that scientists are making great progress in defining the cause of many of the diseases that fall under the umbrella designation ‘Long COVID’. I am also holding out hope that many of the therapeutics we already know and understand will have a significant and immediate impact.
Dear Readers: Please note that each of the topics I am covering in this series is representative of a very large area of study and this overview is designed to provide the reader with an introduction to the topic and timely references rather than serve as a comprehensive review.