Intro to Immune System Dysfunction and Dysregulation
Long COVID Series Part 4.1: Part 4 will be published in three parts that cover an Introduction (4.1), Autoimmune Disease (4.2), Additional Clinical Outcomes of Immune System Dysfunction (4.3).
Intro to Long-COVID Immune System Dysfunction and Dysregulation
As an immunologist by training, I have been following reports of altered immune system function since the early days of SARS-CoV-2. The first report I read that convinced me that SARS-CoV-2 was causing unexpected patterns of immune system activation and suppression was a pre-print published in 2020 from the labs of Diane Mathis and Christophe Benoist (peer reviewed version can be found here) . This team of phenomenal immunologists found that Tregs from patients with COVID-19 looked more like tumor infiltrating Tregs, this is highly unusual for viral infection. Tregs (pronounced like T-Rex but “Reg” instead of Rex) are a type of T cell that when functioning properly keeps us from developing autoimmune disease and helps to fine-tune immune responses. In some patients, these changes persisted after recovery for a few months.
In 2020, data showing changes to the immune system consisted mostly of preliminary reports with a relatively low number of patients. The funding to run large comprehensive studies had not yet come through and it was difficult for scientists to gain access to patient samples. However, immunologists were working hard to try to start defining where we needed to look and the potential dangers of this new virus.
Today, it is clear that SARS-CoV-2 infection alters human immune responses and the long-term impact on the human immune system is starting to be characterized. However, the outcome of persistent immune system dysfunctions and dysregulation may take years to be fully understood. Also, as with any viral infection, it is important to remember that age, severity of infection and genetics will lead to different outcomes for different people. The publications I review here are intended to be representative and not exhaustive. Now, let’s dive into the world of immunology.
Before I continue, a quick note of thanks to my fellow immunologists.
First, I am incredibly proud of our field for immediately tackling these important questions with adherence to excellent science and dedication to development of a mechanistic understanding that is critical for anticipating clinical outcomes. Second, if I have failed to highlight an important area of research or a recent finding please feel free to message me, or leave a comment, as we know our field is extensive and hundreds if not thousands of publications are produced every month, it is possible I may miss something.
What is the Immune System and How Do Immunologists Look for Early Signs of Immune System Dysfunction?
In brief, the immune system is highly complex and distributed throughout the body. It is mostly made up of single cells that are motile (can move through tissues an the blood stream) and distributed throughout the body. Even tissues previously thought to be “immune privileged” all contain immune cells in the tissue or very nearby. T, B and Dendritic cell motility, pathogen finding, and responses are controlled by proteins that they and other non-immune cells secrete or express on their surface.
These specialized proteins (cytokines, chemokines, integrins, and others) are how the cells “talk” to each other and organize a response. When scientists see persistent changes in how immune cells “talk” to each other (lower cytokine levels, epigenetic changes to cells, failure of cells to respond normally to typical signals), that is when we start to get worried. Changes like that usually indicate disease or that a pathogen (virus /bacteria/ fungus) will have an easier time infecting you, and cancerous cells will evade immune system detection more easily.
One way to visualize this is that immune cells are part of an army defending you from pathogens and eliminating cancer cells. When the units or even soldiers can’t communicate, are sending the wrong messages, or the messages are misinterpreted, defenses break down, and even friendly fire can happen (autoimmune disease). The immune system not only protects us from disease but when it malfunctions it can cause disease. It’s difficult to find a disease that does not have some component of immune system involvement. This is why it is critically important that we pay attention to how a new and now widespread virus is impacting population health by altering immune system function.
Why Didn’t We Know Much About Coronaviruses Causing Immune System Issues Before?
SARS-CoV-2 isn’t the first widely distributed coronavirus to infect people. Although it is more severe than many common coronaviruses, there are several shared viral mechanisms between SARS-CoV-2 and other coronaviruses that impact the immune system. The reason the research in this area isn’t particularly robust is that there is a strong correlation between how much of a public health threat a virus poses and the amount of research funding earmarked to study that virus.
The four “common” coronaviruses considered “low pathogenic HCoVs” in wide circulation in the human population are: HCoV-229E, -NL63, -OC43, and -HKU1. A total of about 30 or so coronaviruses have been identified since the 1960s. HCoV-229E, -NL63, -OC43, and -HKU1 are considered mild, they are part of the groups of viruses that cause the common cold. Given that the vast majority of people aren’t worried about the common cold, research funding has generally been directed elsewhere.
“Human Coronavirus-229E, -OC43, -NL63, and -HKU1 (Coronaviridae), 2021 Encyclopedia of Virology” provides an overview of similarities and differences between SARS-CoV-2 and other coronaviruses and outlines several conserved mechanism of virus infection including suppression of host cell production of type 1 interferons, a cytokine or chemical mediator that is a critical pat of the innate immune response.
HCoV- viruses and SARS can trigger other diseases related to dysregulated immune responses, much like SARS-CoV-2, though typically at a much lower rate. In brief, here are some examples:
Possible involvement of HCoV-229E infection in triggering Kawasaki syndrome via serology has been suggested. Kawasaki syndrome is a life threatening disease that is characterized by the immune system suddenly attacking blood vessels, causing systemic vascular issues and cardiac issues in 1 in 5,000 US children under the age of five. It has also been suggested that Kawasaki syndrome may be triggered by SARS-CoV-2.
Several reports of HCoV causing neurological disease can be found here. This review summarizes several diseases triggered by more ‘mild’ HCoVs and details studies from the 1990s showing high levels of HCoV-229E and HCoV-OC43 in plaques of multiple sclerosis patients. Further studies have demonstrated the neurotropism (preferential nervous system infection) of common HCoVs which was also reported early on in SARS-CoV-2 research.
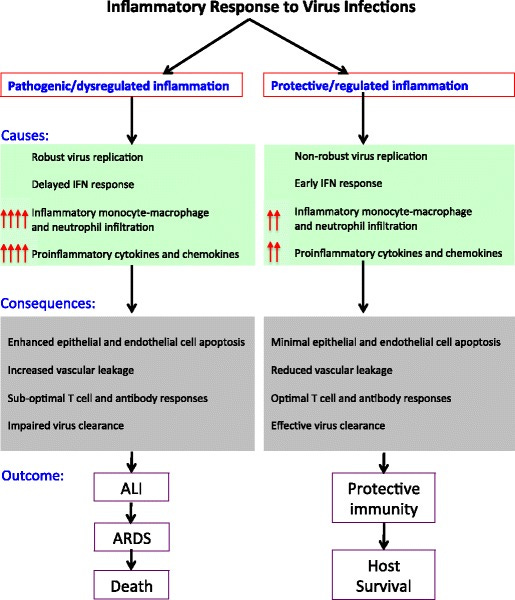
SARS, which did not rise to the level of a pandemic, also demonstrates abortive infection (does not replicate well) of human immune cells leading to altered cytokine profiles and cell death. Indeed, people who died from SARS had evidence of immune system dysregulation, is supported by subsequent studies of animal infection in which multiple species showed suboptimal T cell responses, T cell death, and an accumulation of alternatively activated macrophages (see Figure below).
Fig 2. Schematic representation of protective versus pathogenic inflammatory responses to pathogenic hCoV infections. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology, Seminars in Immunopathology 2017
Given what we know about human coronavirus infection the only thing surprising about SARS-CoV-2 causing immune system dysfunction and dysregulation is the continued (perhaps willful) lack of awareness.
Although there are similarities between HCoV infections and SARS-CoV-2, significant differences have been documented. For example, in a study of patients under 19 years old, white blood cell counts (immune cells) were reduced by ~50% in those testing positive for SARS-CoV-2 relative to those with another coronavirus. Interestingly, the same study found C-reactive protein (CRP), a marker of inflammation, was reduced in younger COVID-19 patients, although it is a marker of severe disease in adult COVID-19. The reduction in CRP was significant relative to children infected with a more mild HCoV. The reason for this is unclear, but indicates a significant difference in immune system behavior in COVID-19 in children relative to HCoV infection.
In general, viral infections can alter immune system function, and there can be periods of lymphopenia post viral infection (reduced white blood cell counts). How long changes last and how clinically meaningful the changes are varies from virus to virus, but in COVID-19, the degree of lymphopenia is significant. For example, in one smaller study this was identified early on with 35% of fully recovered patients (median age: 41, no comorbidities) showed persistent lymphopenia for 2.5 to 3.5 months after recovery.
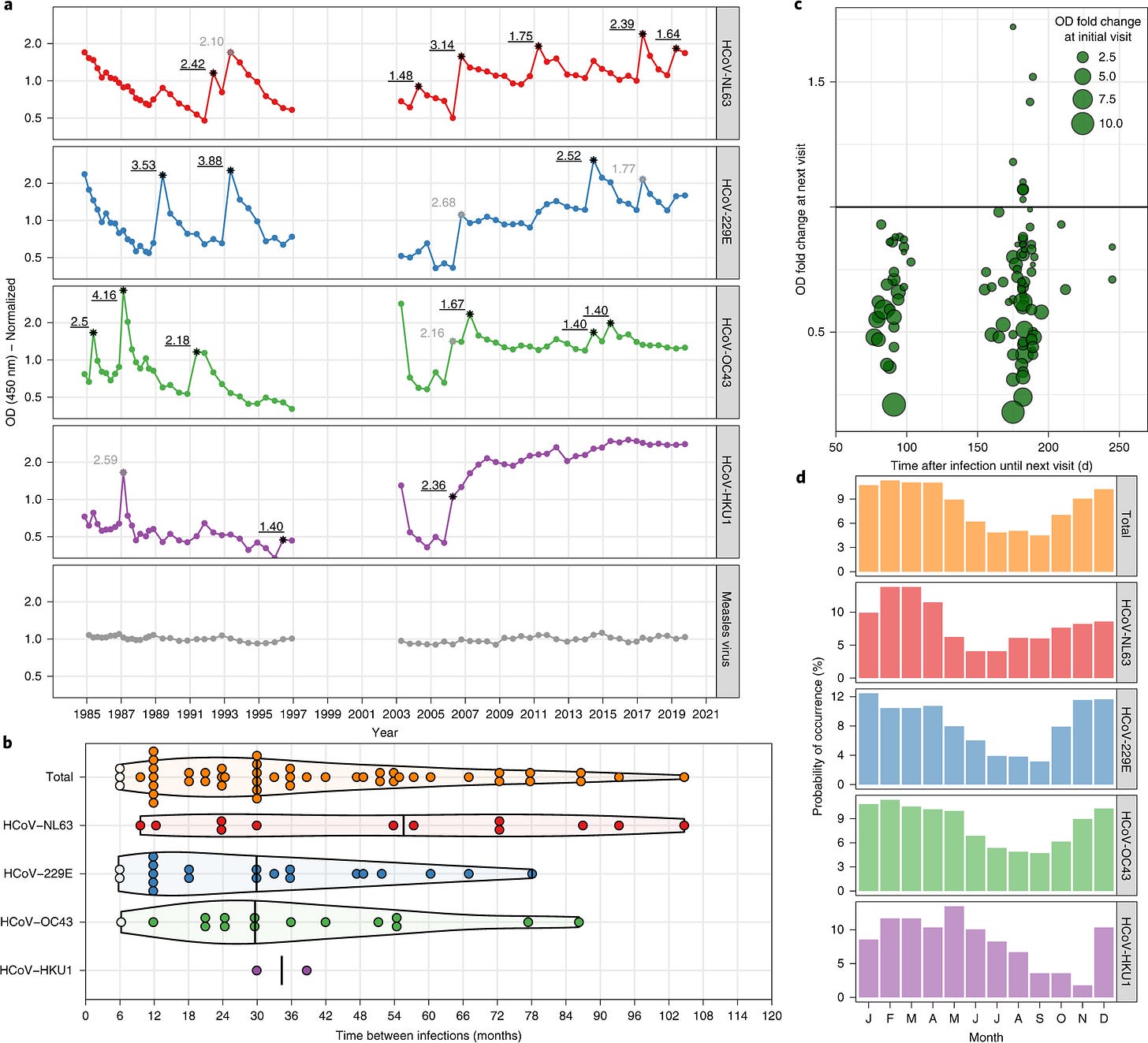
When considering that repeat infections can still cause Long COVID, understanding that repeat infections will persist is important. HCoV-229E infections yield incomplete immunity and reinfections are common. To bring context to this, a study tracking HCoV infection with the most common four viruses in circulation followed 10 individuals for >2,473 months (yes, longer than SARS-CoV-2 has been around) and showed relatively rapid repeat infections (Fig. 1b, below). In contrast, the average adult is only infected with influenza about once every 5 to 7 years. Additionally, their data also shows antibody waning with repeat natural coronavirus infection (Fig. 1d, below).
Seasonal coronavirus protective immunity is short-lasting. Edridge A.W.D. et al. Nature Medicine 2020
Figure 1. a, Example of antibody dynamics of one individual (#9). Connected dots: follow-up intervals <400 d; asterisks: visits with a ≥ 1.40 ELISA OD fold rise in antibodies with the value of the fold change adjacent to the asterisk; black font and underlined: OD fold rises of ≥1.40 classified as infections; gray font: OD fold rises of ≥1.40 suspected of cross-reactivity and not classified as infections. b, The interval between reinfections determined for the ten individuals. White dots: reinfections without an intermediate decrease in antibody levels; black vertical lines: median reinfection times. c, Changes in antibody levels after infection relative to the follow-up interval duration. Each circle represents an infection. Horizontal line: cutoff between increases (>1.0) or decreases (<1.0) in antibody levels at the next follow-up visit. d, The prevalence of infection of the four coronaviruses across different months. The prevalence per month is shown as a percentage of the total number of infections per coronavirus.
This study provides insight into how we might expect SARS-CoV-2 reinfection to behave over the next 30+ years without continued vaccination and public health measures to reduce virus spread. Even if these re-infection rates were the same, because SARS-CoV-2 demonstrates a higher rate of post-acute sequelae (i.e. Long COVID) a public health crisis is slowly building.
Reinfection rates with SARS-CoV-2 are likely higher than the more mild HCoVs due too: 1) high reproduction number (Ro or Re) of SARS-CoV-2, 2) the huge variety of variants that can escape immune protection from prior vaccination or infection, and 3) increasing evidence of immune system dysfunction and dysregulation post COVID-19 may leave people vulnerable to rapid reinfection.
SARS-CoV-2 causing dysregulation (abnormal and often persistent changes to immune responses and/or immune system baseline activity) and dysfunction (inability to produce a strong/normal/expected immune response) have been documented by multiple groups in elegant studies of hundreds of patients.
Here, I will discuss immune system dysregulation in the context autoimmune disease and dysfunction in the context of cancer and reduced capacity to respond to other pathogens. It is also critical to try to understand the duration after infection that immune system issues may persist. In some people, immune system issues may be short-lived (a month or two) while in others SARS-CoV-2 may trigger life-long disease.
Some researchers were able to include comparisons to other viruses. Where possible I will include these comparisons to put a finer point on the similarities and differences between Long COVID and sequelae experienced from infection with other viruses.
In summary, I hope this introduction provided you with the tools to understand the research in this area in the context of how our immune system functions. If you are already in this field, I hope this write up provided some new insights and publications you may not have seen yet. Please note the references are not meant to be exhaustive, but representative of larger bodies of evidence.
Stay tuned for parts 4.2 and 4.3 that will review the overlapping areas of immune system dysregulation and immune system dysfunction. See you there.



Thank you for this. It was dense, but as a non-medical professional I understood almost all of it. Great work! L.