Why Does COVID-19 Impact People Differently?
What have Scientists learned over the last four years about how COVID-19 impacts people differently: Emerging and mostly settled science.
Tornados often leave some homes spared while others are a complete loss. Similarly severe COVID-19 infection doesn’t happen for all people with every infection. Some are more mild and some are more severe. Even people of similar age and health COVID-19 can have a very different impact. This makes it difficult to estimate personal risk of severe infection.
Multiple predictive models for individual infection risk that account for duration of exposure and number of exposures can provide a general guideline for infection risk (modeling below). However, scientists are finding that some genes may be protective from infection and death in some people. These findings may provide clues as to which pathways can be targeted for therapeutic development and possibly improved vaccine strategies. An important disclaimer is that the likelihood of one person carrying a combination of protective genes isn’t guaranteed, even between family members, therefore the “it was just a cold for me” is still a potentially dangerous trope. Here I will review the well understood and emerging research on risk factors for Sars-CoV-2 infection and severe COVID-19.
Quick List:
Strongly Predictive of Infection and Mortality:
Known risk factors of age and comorbidities
HLA genotype
Amount of virus (viral load) one is exposed too
Potential Predictive:
Blood Type (science is still mixed)
Multiple genes across immune system
Miscellaneous genes that may also impact other infection outcomes
Individual probability of getting infected in an average day as a function of the number of exposures and their aggregated duration, according to the model, for various Pmax values. Different Pmax values correspond to individual vulnerability probability, depending on personal immunization and inoculation status. From: Assessing individual risk and the latent transmission of COVID-19 in a population with an interaction-driven temporal model
Amount of Virus During Exposure
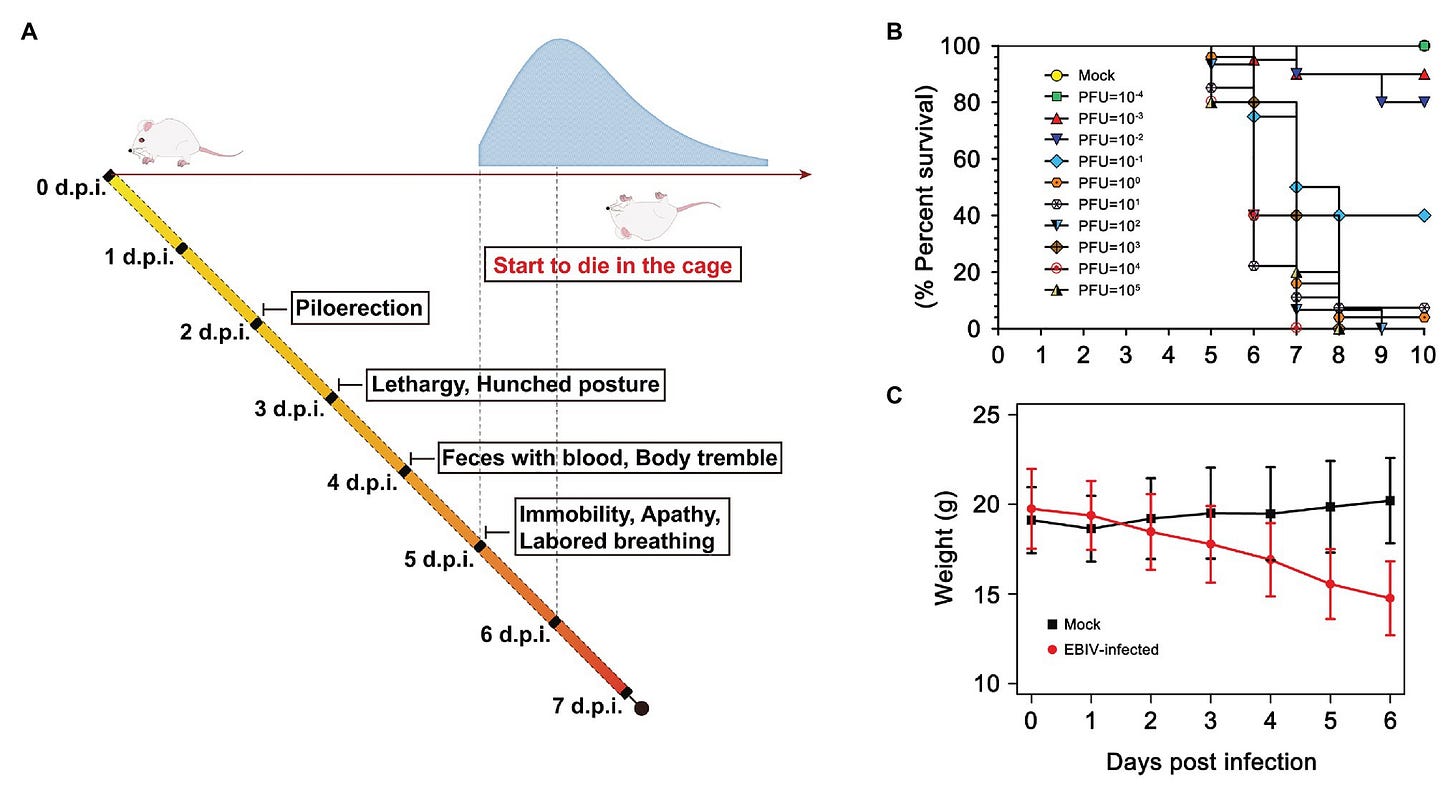
For any pathogen, except perhaps the most deadly, infection and severe disease dynamics are far more complex than infection equating to a poor outcome, even if all other factors are matched. For example, when studying virus infection in the laboratory, you can give the same aged animals, with identical genetic backgrounds the same virus but just in different amounts to create an asymptomatic infection, sublethal, or lethal infection (amount of virus is measured with Plaque Forming Units, PFU)
Clinical illness and survival curve during Ebinur Lake virus (EBIV) infection in male and female BALB/c mice. (A) Behavioral changes. (B) Survival curve. Adult mice (n ≥ 10 per group) were challenged intraperitoneally (i.p) with dose from 10−4 to 105 plaque-forming unit (PFU), (C) Weight change over 6 days when challenged with dose of 10 PFU. From: Pathogenesis and Immune Response of Ebinur Lake Virus: A Newly Identified Orthobunyavirus That Exhibited Strong Virulence in Mice
Also, exposure to a very high amount of virus or repeated, prolonged exposure can overcome what would otherwise be a protective vaccine or immunity from a prior exposure. I am impressed with the contribution of these volunteers for this human study with influenza virus that confirmed findings from animal studies where exposure to higher amounts of virus can lead to disease (dose escalation results table below).
From: Validation of the wild-type influenza A human challenge model H1N1pdMIST: an A(H1N1)pdm09 dose-finding investigational new drug study
This is why minimization of exposure in combination with vaccination has long been practiced in research and hospital settings where direct exposure to infectious viruses is more likely.
Age, Comorbidities, Vaccination, and Immune System Function
Well understood risk factors for severe COVID-19 infection include age, sex, obesity, and pre-existing conditions:
From: Federal Response to COVID-19: Therapeutics Clinical Implementation Guide (Outpatient Administration Guide for Healthcare Providers)
Age: Our immune systems age as we get older. Immune system function starts to decline slowly in our mid-thirties and by 60-65 years immune system function has significantly decreased.
Sex: Hormones can impact immune responses. Men or those with male hormones tend to have a stronger Th1 type response which involves more acute proinflammatory responses. This is why men tend to develop myocarditis at higher rates than women or those with female hormones when infected with SARS-CoV-2. Men (which may include those with predominantly male hormones, not explicitly separated in the data) tend to die more often from SARS-CoV-2 infection. Women (which may include those with predominantly female hormones, not explicitly separated in the data) develop long COVID-19 at higher rates. People who are transgender have the highest rates of long COVID-19.
Vaccination and Infection: A common myth about SARS-CoV-2 is that infection leads to long lasting immunity. Reinfection with the same variant of SARS-CoV-2 has been reported as soon as 21 days, while different variants have been documented to infect people as soon as 14 days after the first infection. Coronaviruses are not known to lead to long-lived immunity after infection and SARS-CoV-2 appears to be no different from its cousins. Someone may have multiple infections before they are hospitalized or develop long COVID-19. This is why vaccination, even after infection, continues to be an important preventative measure.
Additionally, across numerous studies, infection induced immunity is less consistent than vaccination induced immunity. For example, infected people do not always develop protective antibodies post-infection. Sars-CoV-2 neutralizing antibody development is more consistent in vaccinated people. Finally, vaccination plus infection appears to give the most durable long-lasting immune responses. This is likely because the immune system has had more exposure to the pathogen, another training session if you will. Despite additional immune protection this is not a good reason to become infected on purpose. COVID-19 can still cause damage even if vaccinated (though it is reduced on average by vaccination), hybrid immunity still fades, and can be evaded by a different variant.
Emerging Evidence for Protective Factors in SARS-CoV-2 infection
Emerging evidence shows that some people may have a genetic advantage when it comes to avoiding SARS-CoV-2 infection or having more mild disease. The evidence that prior coronavirus infection can be beneficial is mixed, and in some cases prior coronavirus infection may be detrimental.
Human Leukocyte Antigen (HLA) and Infection with Common Coronaviruses
HLA is a protein that the immune system uses to display foreign viral proteins when a cell is infected with a virus. HLA genes are the most diverse gene family in humans which is the reason why it is so difficult to find a bone marrow or organ donor match. It is hypothesized that HLA diversity is selected for by evolution because it plays a large role in the way we fight different pathogens. This may ensure survival or more mild disease among at least some individuals in a population with high HLA diversity. There is even a study that supports the idea that the more different a potential partner’s HLA is from ours, the better they smell to us and that dissimilarity correlates with partnership.
In July 2023, Augsto, D.G. et al. (Nature) showed that there is a strong association between HLA-B*15:01 (a specific genetic version of human HLA) with asymptomatic infection. The protective effect of HLA-B*15:01 was enhanced by also having HLA-DRB1*04:01. This may be because this HLA and corresponding T cell combination have pre-existing memory due to exposure to a small shared bit of protein between SARS-CoV-2 and common corona viruses HKU1-CoV and OC43-CoV.
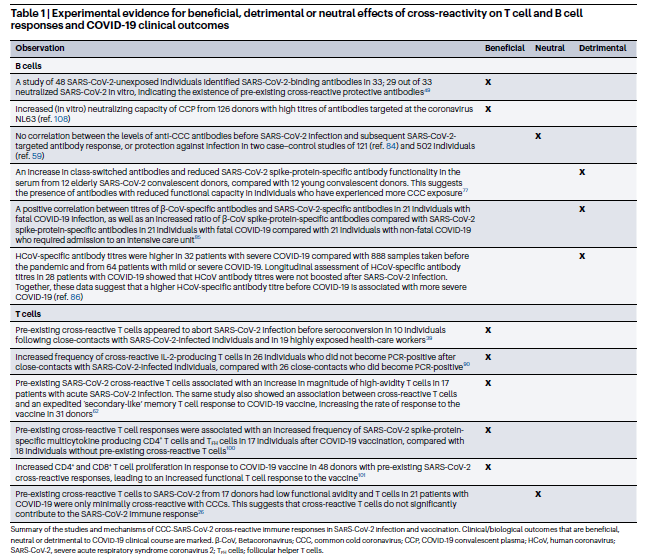
However, the literature is unclear on the benefit of cross-reactive immune responses. Both B cell (antibodies) and T cell (virus infected cell killing and B cell help) cross reactive responses have been shown to be beneficial or neutral, and in the case of B cells more often than T cells be potentially harmful (Figure 1 and Table 1 from Murray, S. M. et al. Nature Reviews Immunology, 2023). Please note that antibody dependent enhancement shown in figure 1d. HAS NOT been shown to occur with Sars-CoV-2 and the authors reference dengue and West Nile viruses as an example of this mechanism.
Figure 1:
Table 1:
Although infection with common rarely lethal coronaviruses may be protective from severe COVID-19 in some cases, it may be detrimental in others. It is likely there are a combination of factors including genetics as indicated by the HLA-B*15:01 genotype findings that dictate degree of benefit.
Genome Wide Association Studies (GWAS) Uncover Genes Linked to Sars-CoV-2 Infection Outcomes
While scientists are finding potential clues using genetic screening, it is important to proceed with caution as results may not be clinically meaningful. In other words, there is not yet a clear “silver bullet” that can consistently predict individual disease outcome or anything that would change the course of treatment or vaccine recommendations.
Blood Type and SARS-CoV-2 Infection and Severity
Blood type association with more or less severe COVID-19 is not yet settled science. Early in the pandemic, a GWAS study indicated that type O blood group may be protective from infection while those with blood type A may be more predisposed to infection. Another study indicating type O might be protective but types AB and B was actually the least protective from intubation once hospitalized. Several studies from around the world indicated protective effect of blood type O; however, populations with higher rates of blood type O do not necessarily have lower death rates. For example, in the United States, Americans of Black and Hispanic heritage have a significantly higher percentage of blood type O relative to Caucasians. However, the rate of death of Black and Hispanic Americans has been higher for the majority of the pandemic, indicating (assuming the same proportions of each population were infected) that if this protective effect exists it may not be able to overcome other factors.
Some studies with controlled patient groups (by race, location, sex), and literature reviews concluded there is no association between blood type and clinical outcome. Recently, a study led by a group at Harvard proposed that previously reported infection susceptibility of those with blood type A may be due to direct interaction of the SARS-CoV-2 receptor binding domain with the blood type A specific sugar molecule (each blood type except O has one or more in the case of blood type AB). In this study, researchers showed that cells from people with blood type A were between 25-50% more susceptible to infection than cells from people with blood type O. Interestingly, infection susceptibility was shown to be variant dependent. Higher rates of infection seen in blood type A cells with the Omicron variant relative to the Delta variant when compared to cells from someone with blood type O.
Currently, the science is not settled as to how protective (or not) any blood type or Rh factor may be, demonstrating the importance of repeating research studies. Regardless of the outcome, the current lack of clear and strong association and the fact that people of all blood types have acquired and died from SARS-CoV-2, I personally do not believe that this can be used as part of a personal risk assessment. Though cumulative evidence does support that blood type A may be more susceptible to infection.
Additional Genetic Factors Uncovered In Genome Wide Association Studies (GWAS)
Whole genome screening projects have been launched in an effort to identify factors that may lead to a more severe COVID-19 infection and death. These screening studies may also help identify pathways or proteins that can be targeted by drugs to reduce COVID-19 severity. Two studies Kousathanas et al. Nature 2022 and Pairo-Castineira, E. Nature, 2023 caught my eye and I will briefly review their findings here.
In a 2022 publication (Kousathanas et al. Nature 2022 ) a group lead by J. Kenneth Baillie, PhD compared the genomes of 7,491 critical COVID-19 patients to 48,400 controls. The team found 23 independent genetic variants that predisposed people to severe COVID-19. Many were components of the immune system and some such as coagulation factor gene F8 and a platelet-derived growth factor receptor-like protein that may play numerous roles in the body (PDGFRL). Interestingly, their study found that the only protective HLA type is HLA-DRB1*04:01, which was noted in the Augusto, D.G. Nature 2023 (discussed above) as an enhancer of projection from symptomatic COVID-19 infection.
In 2023 a group led again by Dr. Baillie published their results (Pairo-Castineira, E. Nature, 2023) from over 24,000 COVID-19 cases with a focus on severe and critical disease (> 6,000 cases) and uncovered 49 genes associated with severe COVID-19 infection. As before, several were related to immune system function such as tumor necrosis factor (TNF) and intracellular signaling such as JAK1. Interestingly, blood type was not identified, but the FUT2 gene associated with secretion of blood type antigens beyond expression on cell surfaces was identified as a genetic variant risk factor. FUT2 is also associated with conferring susceptibility to, or protection from different viral infections as well as chronic conditions Azad et al. 2018 Wellcome Open Res. A chart of genes found and their general role from the article’s Extended Data Fig. 5 (below).
To summarize, you really don’t need to know what a JAK1 kinase is or understand the JAK/STAT pathways to appreciate how rapidly we are moving towards better understanding risk factors for severe COVID-19. Indeed, people have variability in their genetic backgrounds, even blood relatives, that may have combined effects across several genes that can be protective or predispose someone to severe infection. It is still too soon to use these factors as predictors of infection outcome. Therefore, prevention of infection using the tools we have available (vaccination, masks, and social distancing) as well as antivirals such as Paxlovid are still the best course of action.
Historically, this type of screening and targeted drug development is a first. Sars-CoV-2 is a novel virus with unique features and there was little to no pre-established immunity in the population. I am looking forward to this method of exploration being applied in long-COVID such that we can begin to understand this heterogenous condition better, which I suspect may have a genetically linked origins.
Finally, it is still important to avoid being cavalier regarding COVID-19 and repeat infections. We still don’t know if or when people’s “luck” in winning the COVID-19 lottery will run out. Certainly being “spared” from severe disease or Long COVID thus far can lead to a false sense of security.








This is a fantastic read, Merry Christmas!
Wow this is so thorough. I appreciate it!
Do you think we’ll get better therapeutics? To the point the risk for LC and long term damage isn’t as prevalent?